BOD acquires CBD technology, insomnia trial results next two weeks
Disclosure: S3 Consortium Pty Ltd (the Company) and Associated Entities own 3,535,112 BOD shares at the time of publishing this article. The Company has been engaged by BOD to share our commentary on the progress of our Investment in BOD over time.
Our micro-cap cannabis and life sciences Investment Bod Science (ASX:BOD) has clinical trial results incoming.
In as little as two weeks we should know the results from the Phase IIb clinical trial using BOD’s CBD (cannabidiol) product for insomnia sufferers.
After ASX cannabis stocks went peak to trough in the hype cycle between 2019 and 2023 - there’s been a renewed focus by some of these companies on developing clinical evidence their products work.
BOD makes CBD products - and not only does it have clinical trial results due imminently - it has also acquired a new potent delivery technology called Aqua Phase.
In fact, Aqua Phase is so effective that CBD delivered using the technology had around 4 times more bioavailability than the most common delivery method for CBD.
We think it could be the breakthrough that BOD and the global CBD industry have been looking for.
There are two main problems with CBD as we see it.
First, many people swear by CBD - but it's not cheap, sometimes upwards of $200 a bottle.
That's a price point out of reach for many.
Second, some of the claimed benefits associated with taking the CBD are often simply anecdotal.
BOD is aiming to address BOTH of these issues by making CBD work better (which will make it cheaper) AND proving its efficacy (its benefits) in a clinical setting.
With recent data showing BOD’s new technology can make CBD work better, this means a patient wouldn't need to consume as much to achieve the same effect.
And then there’s the clinical trial results we referred to previously - if the results are successful, this could be a major catalyst for the stock.
Roughly 12% of Australian adults suffer from insomnia and the global market for insomnia treatments is expected to be worth US$6.4BN by 2030.
The insomnia clinical trial results have taken a bit longer than expected - the most recent update from BOD is that the results are due “late August” - which is at some stage over the next 2 weeks.
IF BOD is able to succeed, it will be the first over-the-counter cannabis product in Australia and BOD will effectively have 100% of the market share - in a field that has struggled to prove it works.
Success for BOD at this clinical stage also sets the stage for other over-the-counter uses of its product and builds upon a body of evidence for BOD’s products at a clinical level.
Here is where we think that BOD is on the interest cycle for a biotech catalysts:
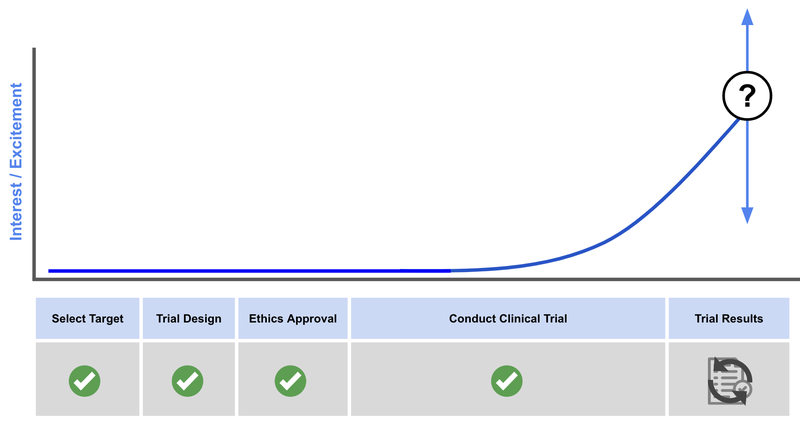
It’s important to note that increased excitement/interest shown on this chart does NOT and will not necessarily correlate to share price increases, which depend on many other factors and broader market conditions.
Ultimately, clinical trials have a binary outcome and have a low chance of success - particularly with cannabis based products that are yet to be proven to improve sleep.
So while there are large potential rewards for companies that can successfully deliver a result in a clinical trial, there is never a guarantee of success.
While we wait for that clinical trial catalyst over the next couple weeks, today’s note will mainly focus on BOD’s new Aqua Phase technology and why it is so important for the company.
ASX:BOD
Bod Science
Last week BOD completed its acquisition of Aqua Phase.
Aqua Phase is a technology that has now been proven to dramatically increase the bioavailability of CBD in the human body - so it's more potent, and more effective.
BOD recently completed two Pharmacokinetic (PK) studies and the results from the first study were a major success.
Aqua Phase delivered CBD had around 4 times more bioavailability than the most common delivery method for CBD.
“Bioavailability” is the ability of the body, in this case the stomach in particular, to absorb a particular substance or active ingredient, like CBD.
BOD can now move to the commercialisation phase: eg. licensing the tech into the multi-billion dollar CBD market, creating new products and potentially even expanding into pharmaceuticals.
The successful PK trial also triggered the completion of the acquisition of the Aqua Phase technology by BOD - which required a payment of £1M ($1.95M) to the UK vendor.
To cover this, BOD raised $1.9M at a placement price of 8 cents, with one free attaching short dated options (expiry date 30 June 2024, exercise price of 10 cents) for every two BOD shares allocated in the placement. These funds are in addition to the $2M cash BOD held at June 30th.
It is also worth noting that the BOD Chairman, David Baker, subscribed for $150,000 of shares in the placement. We like it when directors of companies invest as it is a clear sign they are aligned with other shareholders.
So with the capital raise completed, and a current market cap of ~$13M, BOD can now chase down potential new product and licensing opportunities with Aqua Phase, as we await the insomnia trial results.
We think the main benefits of the Aqua Phase tech are as follows:
- More effective CBD - by increasing the body’s ability to absorb the CBD, the CBD works better.
- Greater margin for CBD companies - because the CBD works better, less active ingredient is needed to achieve the same result, meaning CBD companies won’t need as much material to make their products.
- Better tolerated by patients - because Aqua Phase improves the solubility of CBD - it won’t need to use large amounts of oil, this oil doesn’t always sit well in the stomach.
- New products (licensing) - the improved solubility of CBD makes Aqua Phase well suited to being used in new products like CBD drinks. BOD can licence its tech to companies making these products.
- Improve existing products like pharmaceuticals - beyond CBD products, BOD is exploring the ability of Aqua Phase to improve the body’s absorption of pharmaceuticals - many of these drugs aren’t absorbed well in the stomach and can lead to gastrointestinal problems.
All of these benefits, we hope, lead to further progress by BOD and eventually, the achievement of our BOD Big Bet which is as follows:
Our Big Bet for BOD
“BOD will deliver a minimum 10x return on successfully commercialising at least one application of its new technology that optimises the human body's absorption of cannabinoids.”
NOTE: our “Big Bet” is what we HOPE the ultimate success scenario looks like for this particular Investment over the long term (3+ years). There is a lot of work to be done, many risks involved - just some of which we list in our BOD Investment Memo. Success will require a significant amount of luck. There is no guarantee that our Big Bet will ever come true.
Results of BOD’s Aqua Phase PK Study
BOD conducted a Pharmacokinetic (PK) study to evaluate the bioavailability of cannabis between the Aqua Phase product and regular CBD oil.
The results were very positive, showing that Aqua Phase CBD is 311% better than CBD oil when it comes to total CBD availability.
In simple terms, it works much better.
Here is a chart that shows Aqua Phase compared to a CBD Isolate when it comes to bioavailability.
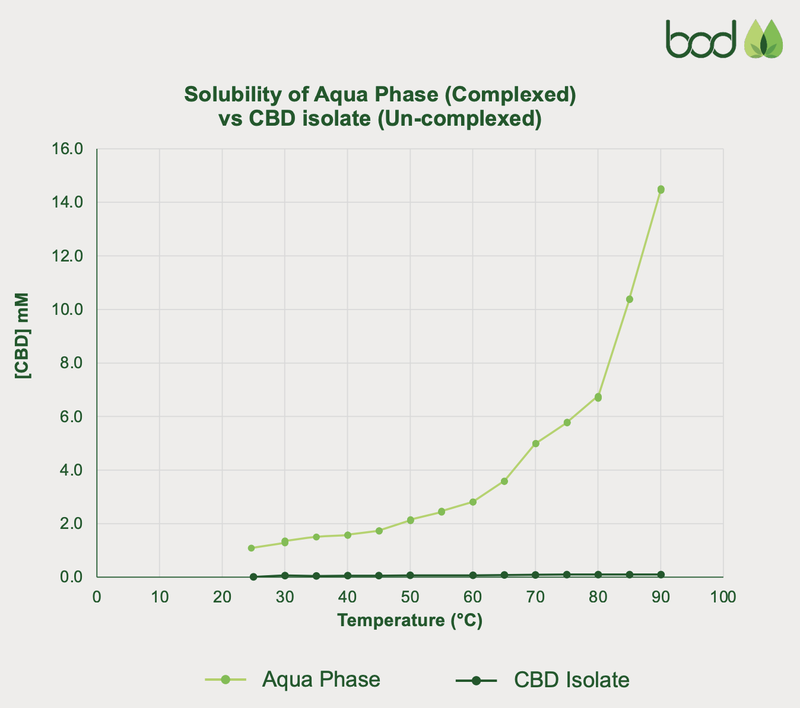
What these results show is that BOD’s product improves how well CBD is absorbed in the body, which unlocks a multitude of opportunities for the company.
As mentioned before, the results announced were the final hurdle before the Aqua Phase acquisition could go through, and BOD has now made the final payment of £1M (~$1.95M) to the vendors to complete the transaction.
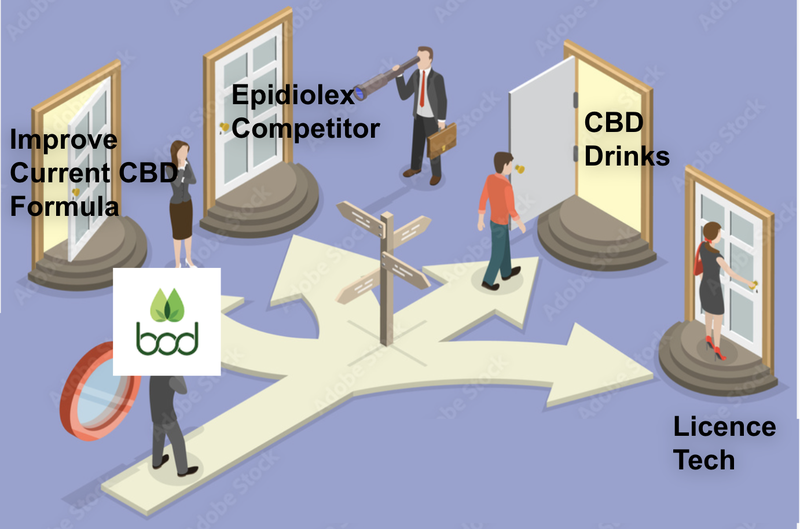
What is BOD’s commercialisation plan for Aqua Phase?
Aqua Phase is a technology that makes cannabis products soluble and more potent, which opens up many commercialisation opportunities for BOD.
In particular, Aqua Phase could make cannabis products cheaper to manufacture, reduce dosage (and as a result cost) for medicines, reduce adverse side effects and improve therapeutic outcomes.
BOD has flagged some potential markets to target, but has yet to pick areas of focus for the technology.
There are many possibilities for Aqua Phase including:
- Licence technology to existing drug manufacturers
- New products, new markets
- Improve BOD’s current product suite
Let’s look at each of these in more detail.
Licence technology to drug manufacturers
Aqua Phase could solve an important solubility problem for drugs with poor side-effect profiles.
When someone ingests a drug, that drug needs to be absorbed by the body to obtain the desired therapeutic effect.
Drugs with poor solubility can have adverse side-effects.
This is where Aqua Phase technology steps in.
BOD has partnered with Kings College in London to see if its Aqua Phase technology can help mitigate the side effects of Clozapine (a widely used psychiatric drug) and Atorvastatin (a cholesterol lowering medication).
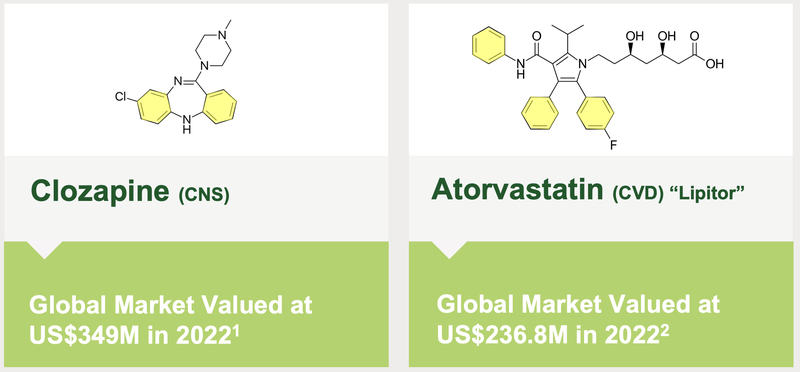
If the data from Kings College is promising, BOD expects “competitive interest” from drug manufacturers in licensing the technology.
New products, new markets
Aqua Phase makes cannabis more soluble.
This could have ramifications for the wider market for cannabis products, and solubility is one of the key limitations for creating cannabis-based beverages.
The US cannabis drink market is estimated to be worth ~US1BN and growing at 54% year on year through 2028 (source).
As an example of how cannabis companies are growing into the beverage space, last week, the largest cannabis company in the world signed an US$85M deal with AB In-bev to grow its portfolio of consumer-based beers.
(Source)

With the largest cannabis company in the world expanding into the beer space, the picture starts to become clear.
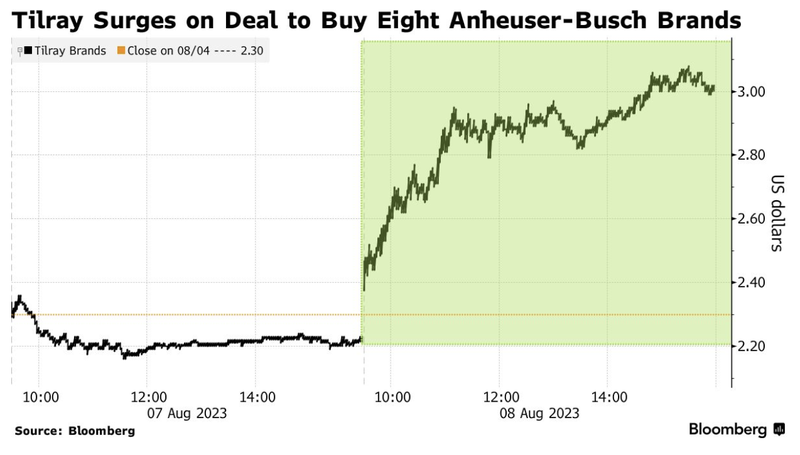
Shares in Tilray surged on the news of the acquisition:
(Source)
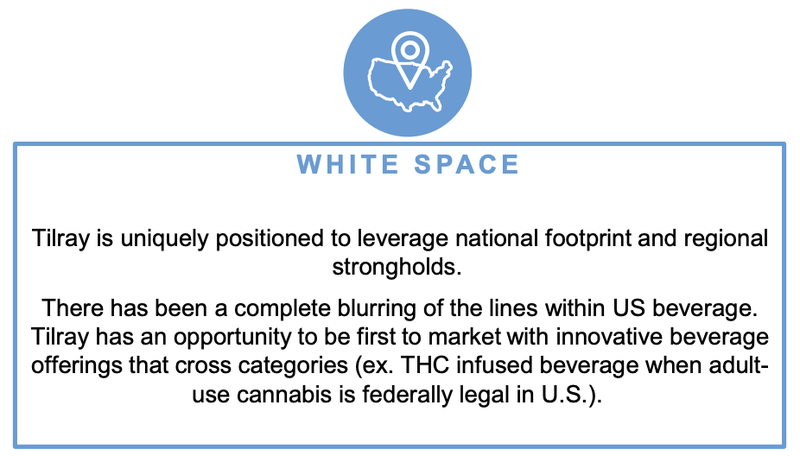
The “Big Bet” for Tilray according to its latest presentation is to be “uniquely positioned” through a national distribution network that provides a stronghold into the THC and CBD infused drinks market:
(Source)
This is how we think Tilray’s strategy will play out:
- Acquire beer brands
- Get foothold into drinks retails and distribution network
- Leverage distribution network for cannabis-infused beverages
- Profit...
The “bet” is that when THC infused beverages are federally legalised in the US, Tilray will have the distribution network to be front and centre of this market.
We think that in a “blue sky scenario” for BOD, its technology could unlock the cannabis drinks product for companies like Tilray and others looking to position for this market.
If BOD could get on the ground floor it could present a valuable opportunity for the company as the US moves towards federal cannabis legalisation.
It’s important to note that we are not suggesting BOD’s product innovations or licensing opportunities are specifically with Tilray or AB In-bev. We just see the Tilray news as an example of what cannabis companies are currently doing to position for the CBD drinks market.
Improve BOD’s current product suite
Looking at opportunities with longer term horizons, BOD can improve its current suite of CBD products and target certain diseases.
Aqua Phase is a technology that can amplify the bioavailability of lipophilic cannabis compounds, which could improve the effectiveness of BOD’s CBD products.
Put simply - more effective CBD dosing, meaning less active ingredient needed and a better tolerance of the treatment.
This means that BOD could produce its own suite of products focused on medical cannabis applications.
In particular, BOD has flagged in the past targeting diseases like child epilepsy.
Child epilepsy is the disease targeted by the biggest cannabis success story - Epidiolex, which was sold to Jazz Pharmaceuticals for $7BN in 2021.
🦉Read more about the child epilepsy moonshot here: BOD’s big bet - will it pay off?
Near-term Insomnia Catalyst
BOD is currently waiting on results for its Phase IIb clinical trial on insomnia.
IF BOD is able to succeed, it will be the first over-the-counter cannabis product in Australia and BOD will effectively have 100% of the market share - in a field that has struggled to prove it works.
Success for BOD at this clinical stage also sets the stage for other over-the-counter uses of its product and builds upon a body of evidence for BOD’s product at a clinical level.
Results are expected by the end of this month.
There have been two other failed attempts to bring an over-the-counter CBD product to market by ASX-listed companies:

Ultimately, clinical trials have a binary outcome and have a low chance of success - particularly with cannabis based products that are yet to be proven to improve sleep.
There is no guarantee of success here.
It’s all about the results now, and we think that excitement / interest levels for BOD’s insomnia trial are at, or close to maximum levels with the trial results right around the corner.
The stock has increased 40-50% in the past two months (since the end of the financial year) and in that time BOD has announced the completion of the clinical trial and set a timeframe for results.

It’s important to note that increased excitement/interest shown on this chart does NOT necessarily correlate to share price increases, which depends on many other factors and broader market conditions.
What’s next for BOD?
Identify key markets to target with Aqua Phase 🔄
With the Aqua Phase acquisition complete we want to see BOD identify some areas of key focus for the technology.
At this early stage we expect BOD to try multiple avenues to commercialisation and then gradually focus on the areas that are gaining the most traction.
Insomnia Trial Results 🔄
BOD has another major catalyst approaching later this month which will see the company release their results from insomnia trials, which is a crucial precursor to TGA approval and potential commercialisation.
Risks
Technical Failure - Insomnia Trial
There is a chance that BOD cannot prove in a clinical setting that its product is more effective than a placebo at treating insomnia.
There have been three other clinical studies like this before, Swinbourne, Cann Group and EcoFibre.
All three trials failed to prove that the product worked compared to a placebo.
Regulatory - Insomnia Trial
If BOD’s clinical trial is successful it could be the only registered over-the-counter CBD product in Australia.
However, if the Australian government decides to change the laws and make it easier for companies to sell over-the-counter CBD products then BOD could lose its regulatory moat.
Market Risk - Insomnia Trial
If BOD’s Phase IIb clinical trial is successful, and approved for Special Access B Scheme by the TGA, it will be able to sell its products in pharmacies over-the-counter.
It does not confer any exclusivity and BOD there is another player that may enter the market if the clinical trial is successful (Avecho).
Funding Risk - General
Small caps always need money to grow.
The challenge is to grow whilst limiting the dilution of existing holders to the upside on success.
BOD held $2M in the bank as of June 30 and just raised $1.9M at 8c. The latest Aqua Phase payment made to complete the deal was ~$1.95M.
Without a major commercialisation / partnership deal or swift uptick in revenue, BOD may need to raise more money to continue its operations at some stage over the coming period.
BOD’s Investment Memo
Below is our Investment Memo for BOD where you can find a short, high level summary of our reasons for Investing.
The ultimate purpose of the memo is to record our current thinking as a benchmark to assess the company's performance against our expectations for the following 12 months.
In our BOD Investment Memo, you’ll find:
- Key Objectives for BOD for the coming year
- Why we are Invested in BOD
- The key risks to our Investment Thesis
- Our Investment plan
General Information Only
S3 Consortium Pty Ltd (S3, ‘we’, ‘us’, ‘our’) (CAR No. 433913) is a corporate authorised representative of LeMessurier Securities Pty Ltd (AFSL No. 296877). The information contained in this article is general information and is for informational purposes only. Any advice is general advice only. Any advice contained in this article does not constitute personal advice and S3 has not taken into consideration your personal objectives, financial situation or needs. Please seek your own independent professional advice before making any financial investment decision. Those persons acting upon information contained in this article do so entirely at their own risk.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.

