US Short Report questions Acadia’s safety - NTI’s competing treatment safety results due within 6 weeks
Disclosure: S3 Consortium Pty Ltd (the Company) and Associated Entities own 8,400,000 NTI shares at the time of publishing this article. The Company has been engaged by NTI to share our commentary on the progress of our Investment in NTI over time.
Neurotech International (ASX:NTI) is currently running a Phase I/II clinical trial for Rett Syndrome.
Results are due within the next 6 weeks.
Phase I/II trial results will reveal if NTI’s treatment is safe/effective.
In the last 72 hours, the supplier of the only currently approved Rett Syndrome treatment (a $6.3BN US company forecasting to make up to ~US$800M/year from treating Retts) was subject to a short seller report...
The short report primarily questioned their treatment's safety.
A perfect backdrop for NTI to release its own, competing Rett Syndrome treatment’s safety results...
And if successful, eat into the analyst estimates of ~US$800M peak revenues per year for treating Rett Syndrome.
Rett Syndrome is a rare genetic neurological and developmental disorder that affects the way the brain develops in very young women.
Rett Syndrome symptoms are horrible and permanently life altering for both the patient and caregivers.
Children with Retts can have: delayed development milestones, loss of motor skills, seizures, intellectual disabilities, as well as sleep disturbance, behavioural problems, social anxiety, poor coordination and vision problems.
Sadly, right now there is no cure for Rett Syndrome.
Rett Syndrome is considered an Orphan Disease and there is only one FDA approved treatment that aims to reduce its impact and severity.
That FDA approved treatment belongs to ASX listed Neuren Pharmaceuticals - the drug is called ‘Daybue’.
In 2018 Neuren closed a licensing deal for Daybue with a NASDAQ listed company called Acadia Pharmaceuticals ($6.3BN market cap).
Between 2020 and 2023, FDA approvals and commercialisation took Neuren’s market cap from ~$100M to a peak of $3.3BN.
Neuren is currently capped at $2.6BN.
Meanwhile, NTI’s Phase I/II clinical trials on its own drug treating Rett Syndrome are due this quarter - which means at some stage over the coming weeks.
NTI is capped at just $97M.
We are Invested in NTI because we think its market cap could go much higher - getting much closer to Neuren’s - IF its Rett Syndrome treatment shows safety/efficacy AND is eventually commercialised.
(which we expect to find out this quarter)
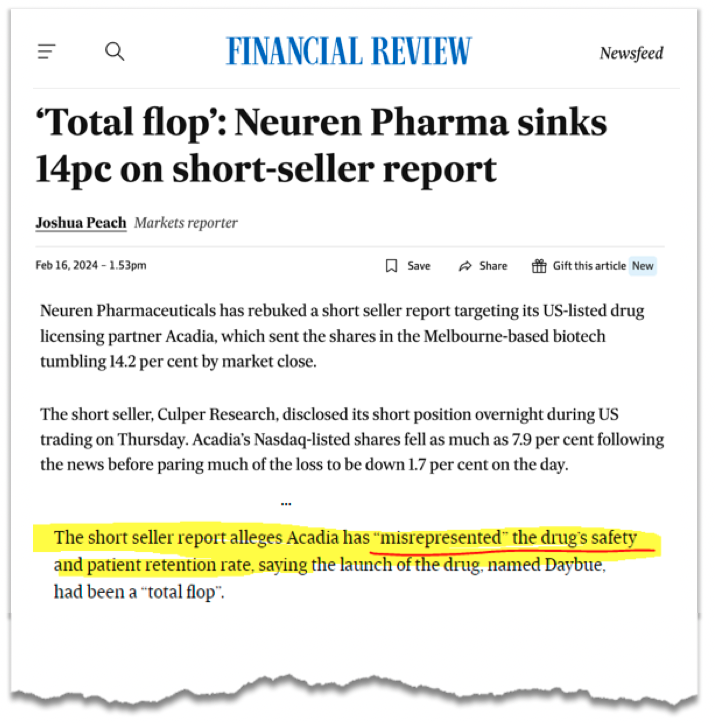
Why did the Neuren share price go down on Friday?
Last Friday we noticed that Neuren’s share price was down ~17% on no news...
Given the similarities to our Investment NTI, we follow the Neuren share price and story relatively closely.
At first, we couldn't figure out why Neuren’s share price was suddenly down, but then we came across a short report on Acadia Pharmaceuticals that had just been released.
Acadia is NASDAQ listed, with a $6.3BN market cap, and has two main assets.
The Rett Syndrome drug licensed from Neuren is the main growth driver for Acadia.
The short report makes the argument that Acadia’s Rett Syndrome drug might not be all it's cracked up to be...
The report alleges that Neuren/Acadia’s treatment causes severe diarrhoea, hospitalisations and little improvement in patients.
(Source)
This could play into the hands of NTI - its early days, but it might end up having a superior treatment to Neuren’s/Acadia’s.
You can access the full short report on Acadia Pharmaceuticals here:
Here is our summary of the main assertions in the 16 page Acadia short report:
- Efficacy concerns - The report alleges that Neuren’s commercial partner Acadia is misrepresenting retention data for its Rett Syndrome product. The short report claims that most patients stop using the treatment after 6 months.
- Safety concerns - The report also alleges that safety data is being misrepresented. The report says virtually all patients get diarrhoea, and ~1 in 10 are hospitalised vs only 13% seeing improvements in their conditions.
- Caregiver feedback is negative - the report also alleges that caregivers have “given up on a drug that often does nothing but cause further pain in loved ones”.
- Insiders leaving the company - The report also points out the fact that in the past 3 months three key insiders including Acadia’s head of R&D, Chief Science Officer, and its General Counsel have left the company - implying insiders are abandoning the company.
- Insurer concerns around price - The report also alleged that insurers were starting to push back on the product considering the safety/efficacy and the US$575,000 price tag for the treatment.
So a large part of the report is pointing out safety concerns for Neuren/Acadia’s Rett Syndrome treatment.
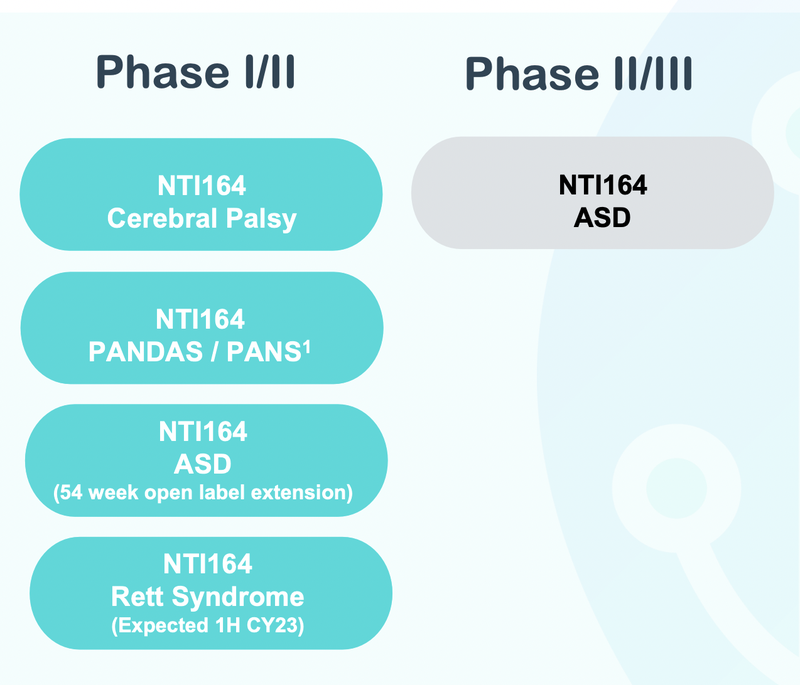
NTI is actually running clinical trials across a number of different neurological diseases including Rett Syndrome, PANDAS/PANS and Autism Spectrum Disorder.
So far NTI has demonstrated in clinical trials that its treatment has almost no safety concerns when being used to treat Autism Spectrum Disorder.
We are hoping that strong safety data is replicated in the results of NTI’s Rett Syndrome clinical trial which we expect to be published in the coming weeks.
This could mean that NTI’s treatment is seen as more favourable by caregivers and could eat into Daybue’s market share for Rett Syndrome treatment if commercialised.
Of course, this is a big “if”.
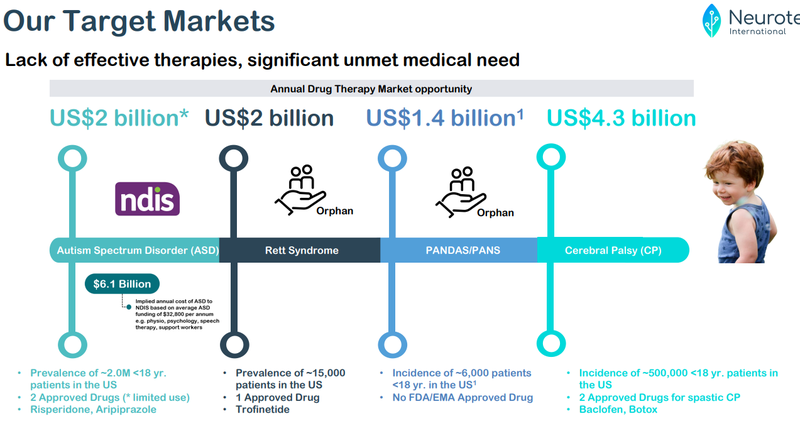
Given the success of Acadia’s commercialisation of Daybue to date, we are hoping that NTI’s own trials will catch the eye of another bigger pharma company eyeing off a slice of the $2BN market share.
At this point, NTI is at an earlier stage in drug trials and approvals and the clinical data will determine whether its treatment is safe and effective.
This is why we think NTI is capped at $97M, a fraction of its much more advanced multi-billion peers.
However, given the issues highlighted in the short report against NTI’s competitor, and the value of being the only successful drug in the market to treat Retts, we like the potential upside in NTI.
Why the short report on Neuren’s Daybue product is a positive for NTI
NTI is developing its own treatment for Rett Syndrome.
NTI’s treatment is materially different to Neuren’s treatment.
Daybue is the only approved Rett Syndrome treatment in the market, and the drug is valued in the billions of dollars.
If there are issues with the drug, as the report alleges, then it makes NTI’s treatment more valuable if the company can get it to market.
At the end of the day it comes down to what the short report alleges.
The report opens with quotes from Daybue caregivers who are reporting negative experiences using the drug.
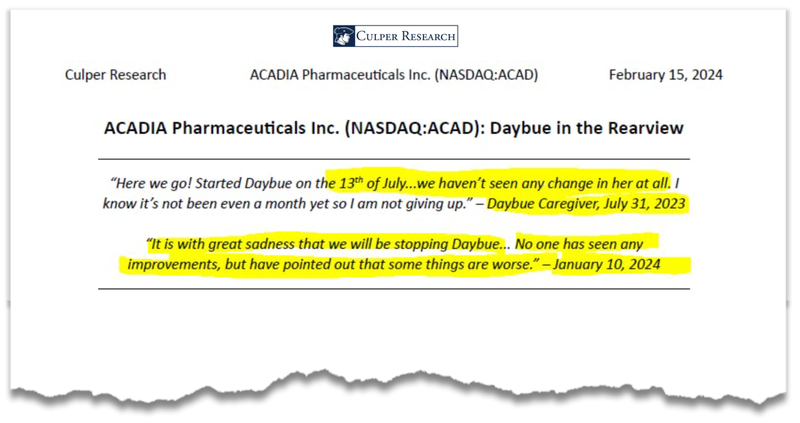
It then expands on why they think the “launch of Daybue” has been, in their words, “a total flop”.

So the basis for the short report is around the safety and efficacy of the Daybue product which is the first and only drug to treat Rett Syndrome out in the market right now.
It's a point we also made when we first Invested in NTI...

(Source)
And that's how NTI could take advantage of this issue with the current drug on the market...
One of the key reasons we Invested in NTI is the strong safety profile of its treatments going into the upcoming clinical trials.
We listed it as one of 7 key reasons why we are Invested in NTI.
Our investment thesis was primarily based on the data from NTI’s Phase I/II ASD (Autism) trial which had shown no serious adverse events across ~52 weeks of use.
Our view was that the safety profile shown by the ASD trial would carry over into the Rett Syndrome trial.
AND that there was a chance NTI could improve on the safety profile of Neuren’s Daybue product.
Why does all that matter?
Because right now there is only one available treatment for Rett Syndrome in the market.
Rett Syndrome is also considered an orphan disease with no cure.
Therefore, there is a significant market opportunity for anyone who can bring a competing treatment to market.
Right now NTI is running a Phase I/II clinical trial which will look at the effects of its NTI164 drug on 14 Rett Syndrome patients.
The trial will have:
- Primary endpoint - based on a Rett syndrome behaviour questionnaire - looking at getting some indication on the efficacy of its treatment.
- Secondary endpoints - looking at safety, adverse events, and measures associated with hand function, motor skills, communication, and quality of life.
So, the results should give us a relatively decent indication on whether or not NTI can improve on Neuren’s safety AND efficacy data.
NTI will likely still need to run further clinical studies before FDA/TGA approval.
BUT any positive data could put NTI on the radar for any potential commercial partners looking to enter the Rett Syndrome market with a product of their own.
Where is NTI relative to Neuren - the only FDA approved Rett Syndrome treatment in the market.
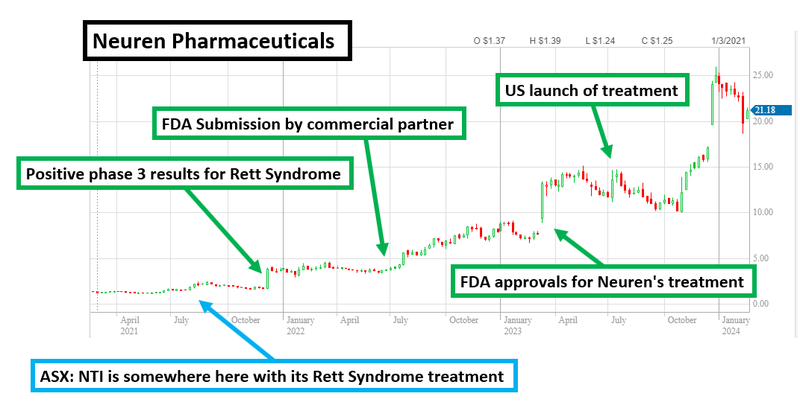
Only a few years ago, Neuren was also at the clinical trial stage - similar to NTI.
Neuren was waiting for results from its Phase III clinical trials and had a share price of ~$1 per share.
After positive Phase III data in October 2021 Neuren’s share price had quadrupled to $4 per share.
Over the next two years Neuren, together with its commercial partner (Acadia) submitted its FDA applications and Neuren’s share price rose to ~$8 per share.
Then in early 2023 the FDA approved Neuren’s Daybue product as the first ever FDA endorsed Rett Syndrome treatment in the market and Neuren’s share price went to highs of over $15 per share.
From the Phase III results to FDA approvals/commercialisation Neuren’s share price went up ~15x.
The past performance is not and should not be taken as an indication of future performance. Caution should be exercised in assessing past performance. This product, like all other financial products, is subject to market forces and unpredictable events that may adversely affect future performance.
NTI is currently capped at $97M and has Phase I/II data coming out in the next few weeks.
We are Invested in NTI because we think the company could follow the same pathway to success that Neuren did over the last ~4 years.
NTI is working on other diseases too...
NTI is focussed on applying its treatment to rare neurological disorders in children, with no or minimal effective treatment.
Success in any one treatment can be highly valued by the market.
A big focus of our note today has been on NTI’s Rett Syndrome clinical trials BUT the company is similarly advanced with trials across three other diseases.
- Autism Spectrum Disorder - NTI has an active Phase II/III clinical trial with results expected inside this quarter.
We covered the upcoming trial results in our most recent NTI note here: NTI autism clinical trial results in next 8 weeks - can it save billions of dollars in NDIS funding needs? - PANDAS/PANS - NTI just recently reported on clinical trial results for its NTI164 treatment in PANDAS/PANS patients. At the moment, there are no regulator approved treatments for PANDAS/PANS, so NTI is hoping to get Orphan Drug Designations for its NTI164 treatment in the US and EU.
- Cerebral Palsy - NTI is currently waiting for human research and ethics approvals in the US/EU so that a Phase I/II clinical trial for paediatric spastic cerebral palsy can start.
We are Invested in NTI to see it get regulatory approvals and then commercialise one of its treatments - this forms the basis for our NTI Big Bet...
Our Big Bet for NTI
“NTI re-rates to a +$500M market cap by successfully advancing one or more of its clinical trial programs through to regulatory approval, partnership/licensing and/or is acquired by a large pharmaceutical company”
NOTE: our “Big Bet” is what we HOPE the ultimate success scenario looks like for this particular Investment over the long term (3+ years). There is a lot of work to be done, many risks involved - just some of which we list in our NTI Investment Memo. Success will require a significant amount of luck. There is no guarantee that our Big Bet will ever come true.
Whats next for NTI
Phase I/II Rett Syndrome clinical trial (results in Q1 CY2024) 🔄
We are hoping NTI can show that its treatment is safer and has similiar (we hope better) efficacy to Neuren’s Daybue treatment.
Especially considering the recent short report drama, any positive news could re-rate NTI’s share price to the upside.
Phase II/III trial for Autism Spectrum Disorder (results in Q1/Early Q2 2024) 🔄
After promising Phase I/II trial results, we see this trial as important from a commercialisation perspective.
What are the risks?
The two key risks to our NTI Investment Thesis are “Clinical trial risk” and “Funding/Dilution risk”.
NTI has clinical trial results due for two target disorders all in the current quarter.
It is important to be aware that clinical trials can be unsuccessful, and investing in early stage biotechs is risky.
It’s possible that the drug/treatment is not considered safe for humans or is ineffective at treating the particular disease.
One or more of NTI’s clinical trials could fail to meet their primary or secondary endpoints, meaning the treatments fail to satisfy the criteria of the studies.
Any clinical trial results, if negative, could hurt the NTI share price.
Clinical trials are also relatively expensive to run, so any failed clinical trials could mean NTI is left with a lower share price and cash balance following the results.
In that scenario NTI would need to raise capital to fund future trials at a discount to already depressed market prices.
However it's also worth noting that NTI had cash and cash equivalents of $4.5M at December 31st 2023 - which the company says is sufficient to complete all active clinical trials as previously indicated to the market.
Background: “Target Disorders” NTI is looking to find treatments for
Autism Spectrum Disorder (ASD)
Autism Spectrum Disorder (ASD) is a developmental disorder which affects social interaction, communication and behaviour. Symptoms appear in early childhood and range in intensity.
- Causes: The exact cause of ASD is unknown, but generally believed to be a combination of genetic and environmental factors.
- Symptoms: Social difficulties, communication challenges, repetitive/restricted behaviours.
- Prevalence: ASD is a common disorder, affecting approximately 1 in 100 children around the world. Prevalence of Autism has been rising in recent times, but the cause is unclear.
- Treatments: There is no current cure for ASD, but early-stage intervention therapy like Applied Behavior Analysis (ABA) and speech therapy can improve outcomes. Medications can also be used for associated symptoms such as anxiety or aggression.
Rett Syndrome
Rett Syndrome is a genetic neurological disorder which primarily impacts females between the ages of 6-18 months, and causes severe cognitive and physical impairments.
- Causes: Rett Syndrome is the result of mutations in the MECP2 gene (located in the X chromosome - hence more prevalent in females). The gene mutation is often sporadic, meaning it is not usually inherited from parents.
- Symptoms: Initial symptoms usually include loss of purposeful hand skills, reduced social engagement and slow growth of limbs. As the syndrome progresses, symptoms can include seizures, repetitive hand movements and intellectual disability.
- Prevalence: Rett syndrome is rare, usually impacting 1 in 10,000 live female births.
- Treatments: There is no cure for Rett Syndrome, but the core focus is to manage symptoms. Symptom management can include medications for anxiety, physical therapy and sometimes devices like limb braces. The recent drug pioneered by Neuren (NEU) targets treatment of Rett Syndrome.
PANDAS - PANS
PANDAS (Paediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections) and PANS (Paediatric Acute-onset Neuropsychiatric Syndrome) are conditions that affect children, resulting in sudden changes in behaviour and mood. PANDAS is specifically linked to strep infections, however PANS can be caused by various infections.
- Causes: PANDAS is thought to be triggered by strep infections in children. Whereas, PANS can be set off by various infections, toxins, and other triggers. Both disorders are autoimmune in nature, where the child’s own cells mistakenly attack healthy brain cells.
- Symptoms: Sudden onset of OCD, tics, anxiety, emotional lability and some motor abnormalities. Symptoms may worsen acutely.
- Prevalence: Research suggests that PANDAS impacts 1 in every 200 children, however prevalence of PANS is less clear.
- Treatments: There is no simple treatment for PANDAS/PANS. Treatment usually involves antibiotics, anti-inflammatories and immunotherapy.
Cerebral Palsy
Cerebral Palsy (CP) is a group of permanent movement neurological disorders, appearing in early childhood. It impacts muscle coordination and motor-neuron skills, but can often impact other body parts too. No two people experience CP in the same way.
- Causes of CP: Cerebral Palsy can be caused by a breadth of reasons, such as brain damage during or shortly after birth. Some risks include premature birth, infections during pregnancy, and complications during labour.
- Symptoms: poor coordination, stiff and weak muscles and tremors. The intensity and severity vary significantly, and these symptoms can often affect multiple limbs.
- Prevalence: It is estimated that 1 in every 323 children are identified to have some form of CP (in the US).
- Treatments: There is no specific treatment for CP. However, CP symptoms are managed by physical therapy, medications (muscle relaxants) and sometimes surgical interventions.
Our NTI Investment Memo
In our NTI Investment Memo you’ll find:
- Key objectives for NTI
- Our long term NTI Big Bet
- Why we are Invested in NTI
- What the key risks to our Investment thesis are
- Our Investment plan
General Information Only
S3 Consortium Pty Ltd (S3, ‘we’, ‘us’, ‘our’) (CAR No. 433913) is a corporate authorised representative of LeMessurier Securities Pty Ltd (AFSL No. 296877). The information contained in this article is general information and is for informational purposes only. Any advice is general advice only. Any advice contained in this article does not constitute personal advice and S3 has not taken into consideration your personal objectives, financial situation or needs. Please seek your own independent professional advice before making any financial investment decision. Those persons acting upon information contained in this article do so entirely at their own risk.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.






