Noxopharm shares surge on appointment of high profile personnel to support NOX66 clinical trials
Hey! Looks like you have stumbled on the section of our website where we have archived articles from our old business model.
In 2019 the original founding team returned to run Next Investors, we changed our business model to only write about stocks we carefully research and are invested in for the long term.
The below articles were written under our previous business model. We have kept these articles online here for your reference.
Our new mission is to build a high performing ASX micro cap investment portfolio and share our research, analysis and investment strategy with our readers.
Click Here to View Latest Articles
As Noxopharm (ASX:NOX) prepares to embark on clinical trials in relation to its NOX66 product, the group has appointed three prominent medical consultants to assist in shaping the program and providing hands-on experience in the course of the trials.
While one Professor and two Associate Professors have been appointed, it is important to note that these medical advisors are more than mere academics, having substantial practical experience in their fields which are relevant to the trials being undertaken.
As NOX’s Chief Executive, Doctor Graham Kelly, explained “We wanted advisors who will be active participants in the development of NOX66, including running some of our clinical studies, and not just advising from a distance”.
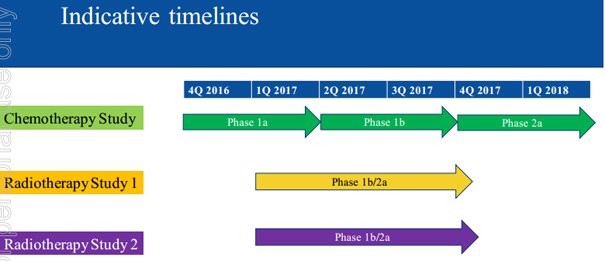
Kelly stressed that the proposed program of five clinical studies is a considerable undertaking that will require the backing of an experienced advisory panel comprising Australian medical oncologists and radiation oncologists, most of whom will be active participants in the clinical program.
Past experience with Noxopharm should prove beneficial
Professor Paul de Souza holds the foundation chair in Medical Oncology, School of Medicine, Western Sydney University. He will advise NOX on both its clinical trial and drug development strategies, as well as chairing the company’s medical advisory panel.
His experience will be invaluable given he has been involved with the company’s core technologies, having conducted the first phase 1B clinical study of idronoxil as well as a number of laboratory studies.
Associate Professors, Thomas Eade and George Hruby have been appointed as radiation oncology consultants. Both hold senior staff positions at Royal North Shore Hospital in Sydney, and have clinical academic positions in the Faculty of Medicine at the University of Sydney.
An intimate understanding of these areas of medicine will be crucial as the group pursues the development of NOX66 as an adjunct therapy for both chemotherapy and radiotherapy, particularly with a view to sensitising cancer cells to the extent that low dosages of chemotherapy or radiotherapy will be sufficient to produce meaningful clinical responses when no response would be anticipated.
Where is NOX66 heading
As a backdrop, the program is designed to test NOX66 across a range of cancer types and under differing treatment regimens with the objective of providing timely proof of concept and guidance for registration studies.
NOX is still in the early stages of its program and thus investors should seek professional financial advice if considering this stock for their portfolio.
The cancers being tested involve prostate, breast, ovary, lung and head and neck carcinomas. Proof of concept clinical data is expected to be forthcoming in the next 12 months.
NOX is tracking well to 2018.
The studies involve patients with late stage cancers for whom there are no remaining standard therapeutic options, are open studies, and involve between 10 and 15 patients.
From a broader perspective, NOX has a primary focus on the development of drugs to address the problem of drug resistance in cancer cells, a major hurdle facing improved survival prospects for cancer patients.
NOX66 designed to protect idronoxil
Looking specifically at NOX66, it is an innovative dosage formulation of the experimental anti-cancer drug, idronoxil, developed to protect idronoxil from being inactivated in the human body by Phase 2 metabolism.
The purpose of NOX66 is to ensure that most idronoxil administered remains in an active form allowing it to work by cancelling pro-survival mechanisms in cancer cells regulated by sphingosine-1 phosphate that allow the cells to resist the killing effects of chemotherapies and radiotherapy.
Today’s news has been well received with NOX’s shares hitting a high of 50 cents in morning trading, representing an increase of 14% compared with yesterday’s closing price of 44 cents.
It should be noted that share trading patterns should not be used as the basis for an investment as they may or may not be replicated. Those considering this stock should seek independent financial advice.
General Information Only
S3 Consortium Pty Ltd (S3, ‘we’, ‘us’, ‘our’) (CAR No. 433913) is a corporate authorised representative of LeMessurier Securities Pty Ltd (AFSL No. 296877). The information contained in this article is general information and is for informational purposes only. Any advice is general advice only. Any advice contained in this article does not constitute personal advice and S3 has not taken into consideration your personal objectives, financial situation or needs. Please seek your own independent professional advice before making any financial investment decision. Those persons acting upon information contained in this article do so entirely at their own risk.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.