Respiri receives CE Mark approval
Published 12-APR-2017 16:05 P.M.
|
3 minute read
Hey! Looks like you have stumbled on the section of our website where we have archived articles from our old business model.
In 2019 the original founding team returned to run Next Investors, we changed our business model to only write about stocks we carefully research and are invested in for the long term.
The below articles were written under our previous business model. We have kept these articles online here for your reference.
Our new mission is to build a high performing ASX micro cap investment portfolio and share our research, analysis and investment strategy with our readers.
Click Here to View Latest Articles
Respiri (ASX: RSH), a leading developer of technologies to assist in remote monitoring of chronic disease symptoms, has been awarded the CE Mark (Conformite Europeene) approval for the proprietary Next Generation AirSonea home monitoring device, which provides users with a robust and security compliant objective asthma monitoring and measurement system.
Highlighting the potential revenue generating significance of this development, RSH’s Chairman, Leon L’Huillier said, “The European approval to market AirSonea was important to advance partner discussions, and it provides the commercial opportunity to pilot and test market the products from current inventory, a significant outcome in that it allows for the sale of the devices into the UK and Europe”.
However, how much revenue will be generated is speculative and investors should seek professional financial advice if considering this stock for their portfolio.
As a backdrop, the first generation AirSonea technology and software received regulatory approval in the UK, Europe and Australia as a Class I medical device. The world leading, updated AirSonea is a Class 11a medical device and as such, underwent a greater level of assessment by a third-party to achieve the updated CE Mark.
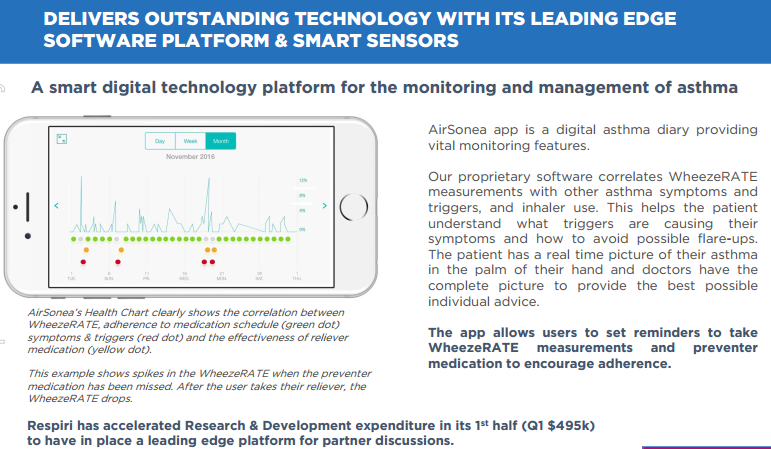
The product also has a number of new technological features. Harnessing the processing power of the smartphone, the proprietary acoustic respiratory monitoring (ARM) technology is now housed in the app rather than the cloud so patients can measure their wheeze symptom without an Internet connection.
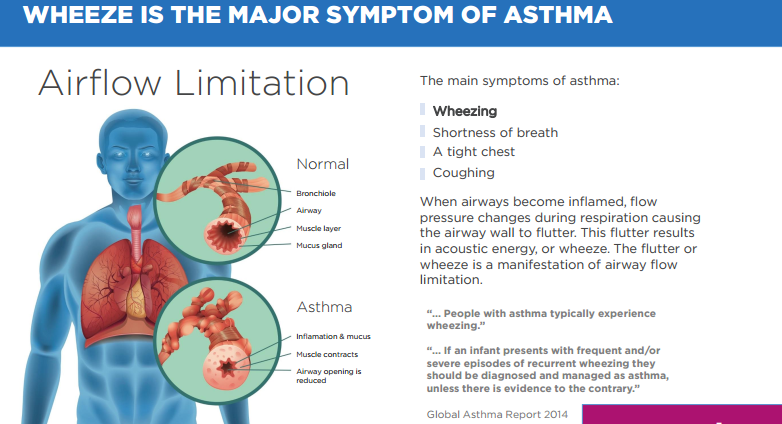
Data is then automatically uploaded to the cloud when a Wi-Fi connection is established. Patient data, including time and date stamped wheeze measurements, medication use and symptoms, as well as triggers are securely stored in the cloud for review by health professionals who until now relied on a patient’s memory or handwritten notes relating to their asthma management plan.
CE Mark approval paves the way for revenue growth
This assessment and approval is a critical step which validates the progress made to improve the AirSonea technology to be the world leading, gold standard in non-invasive, objective monitoring of asthma.
CE Mark approval also completes the regulatory process to the highest level, allowing RSH to advance partnership discussions in one of the three biggest medical device markets in the world.
While many product developers in the medical devices space struggle over long periods of time to gain approvals, RSH is now in a position where it can generate income from a product that doesn’t require a doctor’s prescription and is available on an over-the-counter basis.
Importantly, RSH has first mover advantage in targeting a major unmet consumer need with its primary market being parents and carers of children who cannot perform lung function tests.
Even from a broader perspective there is an increasing recognition of the benefits of mHealth solutions at a time when demand for medical resources is outstripping supply.
Having the capacity to monitor and treat a condition from remote locations without visiting a medical practice is convenient for users and in some cases life-saving in terms of being able to quickly retrieve and communicate information, as well as responding with the appropriate medication or treatment.
It is worth noting that there is general industry support for such devices as government’s struggle to develop infrastructure and bolster human resources to handle the increased demand for health services.
General Information Only
This material has been prepared by Jason Price. Jason Price is an authorised representative (AR 000296877) of 62 Consulting Pty Limited (ABN 88 664 809 303) (AFSL 548573) (62C), and a Director of S3 Consortium Pty Ltd (trading as StocksDigital).
This material is general advice only and is not an offer for the purchase or sale of any financial product or service. The material is not intended to provide you with personal financial or tax advice and does not take into account your personal objectives, financial situation or needs. Although we believe that the material is correct, no warranty of accuracy, reliability or completeness is given, except for liability under statute which cannot be excluded. Please note that past performance may not be indicative of future performance and that no guarantee of performance, the return of capital or a particular rate of return is given by 62C, Jason Price, StocksDigital, any of their related body corporates or any other person. To the maximum extent possible, 62C, Jason Price, StocksDigital, their related body corporates or any other person do not accept any liability for any statement in this material.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.




