Neurotech Demonstrates Potential Benefits for Management of Multiple Sclerosis Disease
Hey! Looks like you have stumbled on the section of our website where we have archived articles from our old business model.
In 2019 the original founding team returned to run Next Investors, we changed our business model to only write about stocks we carefully research and are invested in for the long term.
The below articles were written under our previous business model. We have kept these articles online here for your reference.
Our new mission is to build a high performing ASX micro cap investment portfolio and share our research, analysis and investment strategy with our readers.
Click Here to View Latest Articles
Neurotech International Limited (ASX:NTI) has announced the continuation of its pre-clinical program in neuro-inflammatory disease models in-line with its research and development pathway.
Initial in vitro studies were carried out in collaboration with the internationally recognised Neurodevelopment in Health and Disease Laboratory at RMIT University to assess the effects of the lead NTI/Dolce strains on key neuro-markets that are used in assessing the onset and progression of Multiple Sclerosis (MS).
Summarised in the table below, the results reconfirm the powerful neuro-modulatory, neuro-regulatory and neuro anti-inflammatory properties of the novel NTI/Dolce Strains compared to CBD alone in supressing the neuro-inflammatory markers, GM-CSF and TNF-alpha, in a neural cell-line culture.
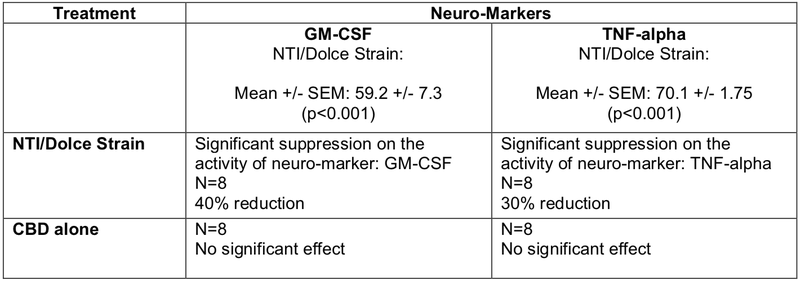
These preclinical studies will pave the way for further expansion and analysis of other neuro-markers involved in MS.
There is a strong medical need for treatment alternatives which include the potential of utilising the NTI/Dolce Strains given they have < 0.3% THC and a full spectrum cannabinoid profile that has already demonstrated the activation of alternative anti-inflammatory neuro modulatory pathways that differ considerably to CBD alone products.
Key neuro-markers involved in the onset and progression of MS include:
- Granulocyte Granulocyte-macrophage colony-stimulating factor (GM-CSF)
- Tumour Necrosis Factor (TNF-alpha)
- Interferon (IFN)
- Interleukins (IL-2)
NTI is committed to the development of a solid scientific portfolio for the expansion of application and use of the NTI/Dolce Strains beyond autism. Further preclinical studies will determine mode of action and safety to design and undertake a Phase I/II clinical study in MS.
“There are a number of very powerful neuro-markers that are currently being used to assess disease onset and progression,” said Brian Leedman, Chairman of Neurotech. “To be able to suppress or regulate these markers may be very beneficial in the overall disease management”.
General Information Only
This material has been prepared by Jason Price. Jason Price is an authorised representative (AR 000296877) of 62 Consulting Pty Limited (ABN 88 664 809 303) (AFSL 548573) (62C), and a Director of S3 Consortium Pty Ltd (trading as StocksDigital).
This material is general advice only and is not an offer for the purchase or sale of any financial product or service. The material is not intended to provide you with personal financial or tax advice and does not take into account your personal objectives, financial situation or needs. Although we believe that the material is correct, no warranty of accuracy, reliability or completeness is given, except for liability under statute which cannot be excluded. Please note that past performance may not be indicative of future performance and that no guarantee of performance, the return of capital or a particular rate of return is given by 62C, Jason Price, StocksDigital, any of their related body corporates or any other person. To the maximum extent possible, 62C, Jason Price, StocksDigital, their related body corporates or any other person do not accept any liability for any statement in this material.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.






