MyFiziq teams up with Evolt to launch new age app
Hey! Looks like you have stumbled on the section of our website where we have archived articles from our old business model.
In 2019 the original founding team returned to run Next Investors, we changed our business model to only write about stocks we carefully research and are invested in for the long term.
The below articles were written under our previous business model. We have kept these articles online here for your reference.
Our new mission is to build a high performing ASX micro cap investment portfolio and share our research, analysis and investment strategy with our readers.
Click Here to View Latest Articles
MyFiziq Limited (ASX: MYQ) has provided an update on the release of the Evolt360 application which is now available in the Apple App store.
Evolt is an FDA approved medical grade device/machine-based intelligent body scanning technology purchased and supported by health, fitness, and wellness companies including but not limited to F45, Anytime Fitness, HCF and KPMG.
These companies and many more are looking to provide greater insight into the health of their members and employees.
The scans performed by the technology are made available to consumers for between $30 and $50 per scan depending on the type of scan performed and level of membership.
Evolt has released the much anticipated Evolt360 application with in-app body scanning using the MyFiziq technology.
The MyFiziq technology is intended to extend the Evolt reach into the hands of their 500,000 consumers at home.
With the new integration, customers of Evolt will be able to download access to the MyFiziq technology, via the Evolt app, which is available in the Apple App Store.
The Evolt application with the MyFiziq in-app scanning capability is being released on iOS initially, with Android to follow.
In addition to the body tracking capabilities, Evolt will be embedding its biometric data into the machine learning data with MyFiziq within the application, delivering a broader data set than has been made available in any application of its kind to date.
Tracks quality of activity, body composition and nutrition
Evolt has developed a unique end-to-end solution that tracks detailed changes in the quality of the activity, body composition and nutrition, as well as sophisticated data analytics for its user’s body changes.
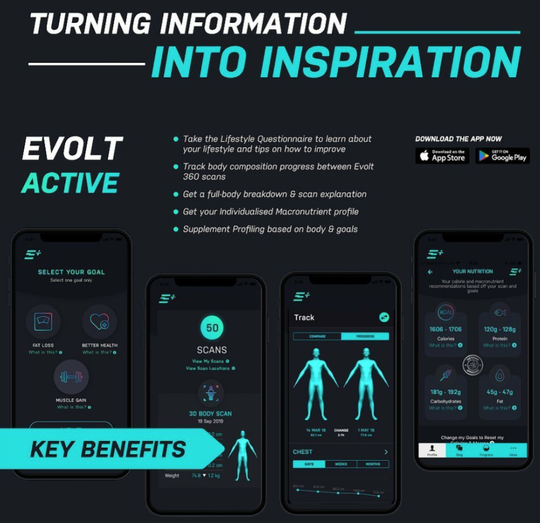
With the added functionality of tracking circumference change as an additional data set, Evolt is inspiring and educating its users with real-time data to drive and assist in the delivery of personal goals.
In just a few short years, Evolt has become a global player, securing contracts across multiple verticals, from health insurers, health, and fitness retailers to government bodies and some of the world’s largest gym chains.
Targeting robust revenues in first 12 months
Highlighting the potential revenue generation from incorporating Evolt into MyFiziq’s product offering, chief executive Vlado Bosanac said, “The launch of Evolt has been much awaited both by MyFiziq and our shareholders.
"Evolt has committed to targeting an initial 100,000 active users onto the joint offering in the first 12 months and MyFiziq will receive $2.99 per month per user. This will mark the release of our second consumer application.
"If the initial target user numbers are achieved, it would see the Company meet an initial break-even position, based on current monthly burn. The application has been through rigorous testing in readiness for this launch. I am pleased to say the application is performing well on all fronts. The look and feel of the application is a credit to both teams. We are looking forward to supporting Evolt with its launch, users, downloads, and usage. As Evolt has been granted an FDA medical device approval, we believe this will bring a great deal of interest to both companies as we co-promote this unique and comprehensive capability to multiple business verticals, both current and new.
"The FDA medical device approval held by Evolt is of particular benefit to both companies as we share a common interest in distributing the solution to governments, medical organisations, and insurers. The FDA approval brings validity to the device and its functionality is often required in such organisations, when looking to use the device as part of a decision-making process rather than a monitoring device.”
General Information Only
This material has been prepared by Jason Price. Jason Price is an authorised representative (AR 000296877) of 62 Consulting Pty Limited (ABN 88 664 809 303) (AFSL 548573) (62C), and a Director of S3 Consortium Pty Ltd (trading as StocksDigital).
This material is general advice only and is not an offer for the purchase or sale of any financial product or service. The material is not intended to provide you with personal financial or tax advice and does not take into account your personal objectives, financial situation or needs. Although we believe that the material is correct, no warranty of accuracy, reliability or completeness is given, except for liability under statute which cannot be excluded. Please note that past performance may not be indicative of future performance and that no guarantee of performance, the return of capital or a particular rate of return is given by 62C, Jason Price, StocksDigital, any of their related body corporates or any other person. To the maximum extent possible, 62C, Jason Price, StocksDigital, their related body corporates or any other person do not accept any liability for any statement in this material.
Conflicts of Interest Notice
S3 and its associated entities may hold investments in companies featured in its articles, including through being paid in the securities of the companies we provide commentary on. We disclose the securities held in relation to a particular company that we provide commentary on. Refer to our Disclosure Policy for information on our self-imposed trading blackouts, hold conditions and de-risking (sell conditions) which seek to mitigate against any potential conflicts of interest.
Publication Notice and Disclaimer
The information contained in this article is current as at the publication date. At the time of publishing, the information contained in this article is based on sources which are available in the public domain that we consider to be reliable, and our own analysis of those sources. The views of the author may not reflect the views of the AFSL holder. Any decision by you to purchase securities in the companies featured in this article should be done so after you have sought your own independent professional advice regarding this information and made your own inquiries as to the validity of any information in this article.
Any forward-looking statements contained in this article are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results or performance of companies featured to differ materially from those expressed in the statements contained in this article. S3 cannot and does not give any assurance that the results or performance expressed or implied by any forward-looking statements contained in this article will actually occur and readers are cautioned not to put undue reliance on forward-looking statements.
This article may include references to our past investing performance. Past performance is not a reliable indicator of our future investing performance.